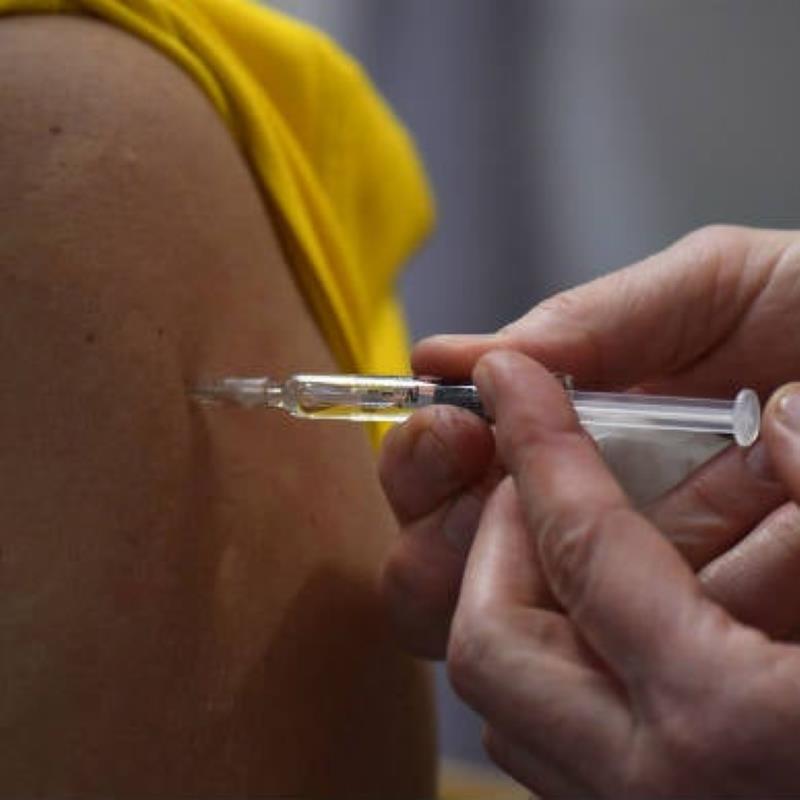
Pfizer and Moderna have asked for emergency approvals of their COVID-19 vaccine candidates in Europe, the region’s health regulatory agency announced on Tuesday. Both companies filed for conditional marketing authorizations, the European Medicines Agency (EMA) said. The agency said a decision could be issued “within weeks, depending on whether the data submitted are sufficiently robust and complete to show the quality, safety and effectiveness…


More Stories
Congress’ new crop of diverse Republicans face option of joining Democrat-leaning minority caucuses. Will they?
Defense bill battle showcases 2024 GOP hopefuls
Attorney General William Barr is reportedly brushing off Trump’s attacks over Hunter Biden investigation as a ‘deposed king ranting’